
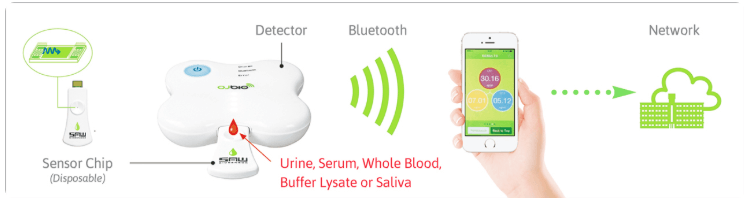
Through converging the expertise and technologies of its two parent companies,OJ-Bio developed a biosensor technology capable of measuring biomarkers on biochips.
The Xtalline product range represents the state-of-the-art in mobileenabled testing and monitoring.
Principles of SAW Measurement
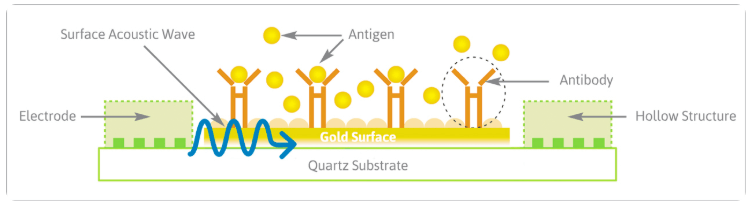
No complicated equipment is needed for the analysis, its purely electronics. The surface acoustic wave (SAW) chips, normally found for a different purpose in mobile phones and other electronic devices, are converted into biosensors. Antibodies on our bio-chips are held in the correct orientation to react specifically with target biomarkers. Analyte binding causes a shift in the phase angle of the SAW passing across the chip surface which is translated into an electronic signal. The test result is then displayed on a mobile phone app or any health care record system. Our technology – for the first time – enables SAW technology to be used to its full potential with biological samples and the resulting devices are able to detect biomarkers in blood, urine or saliva.
Features and Benefits
Xtalline brings the benefits of portability, minimal training and maintenance allowing testing and monitoring to be undertaken anywhere by anyone - even patients themselves. It is an easily handled and cost-effective device allowing rapid and reliable tests at the point-of-use. The quantitative results allow precise decisions for diagnosis and monitoring improving health and wellness.

Xtalline enables multiple tests and applications in one device, in trials it has proven capability for a range of diseases. OJ-Bio's lead application is a biochip that can measure the biomarker C-Reactive Protein (CRP). CRP is a protein biomarker of inflammatory disease allowing determination of whether an infection is caused by bacteria and whether or not antibiotics are required. Thus, it enables an immediate decision at the point-of-use, encourages appropriate antibiotic prescription, and helps to decrease antibiotic resistance. The Xtalline-CRP product will be launched in 2016. Additionally, clinical feasibility studies are completed for other applications including periodontal disease, flu and HIV.
OJ-Bio welcomes licensing of its technology as well as partnerships to find new and additional application for its technology to develop new products and discover new commercial opportunities. We have a strong intellectual property portfolio in support of our technology platform and full manufacturing capabilities to ISO13485.
